For decades, modern medicine was built on male biology as the default and female biology as a deviation, a problem rooted in the long-standing exclusion of women from medical research.
Now, a Swedish startup is here to change all this.
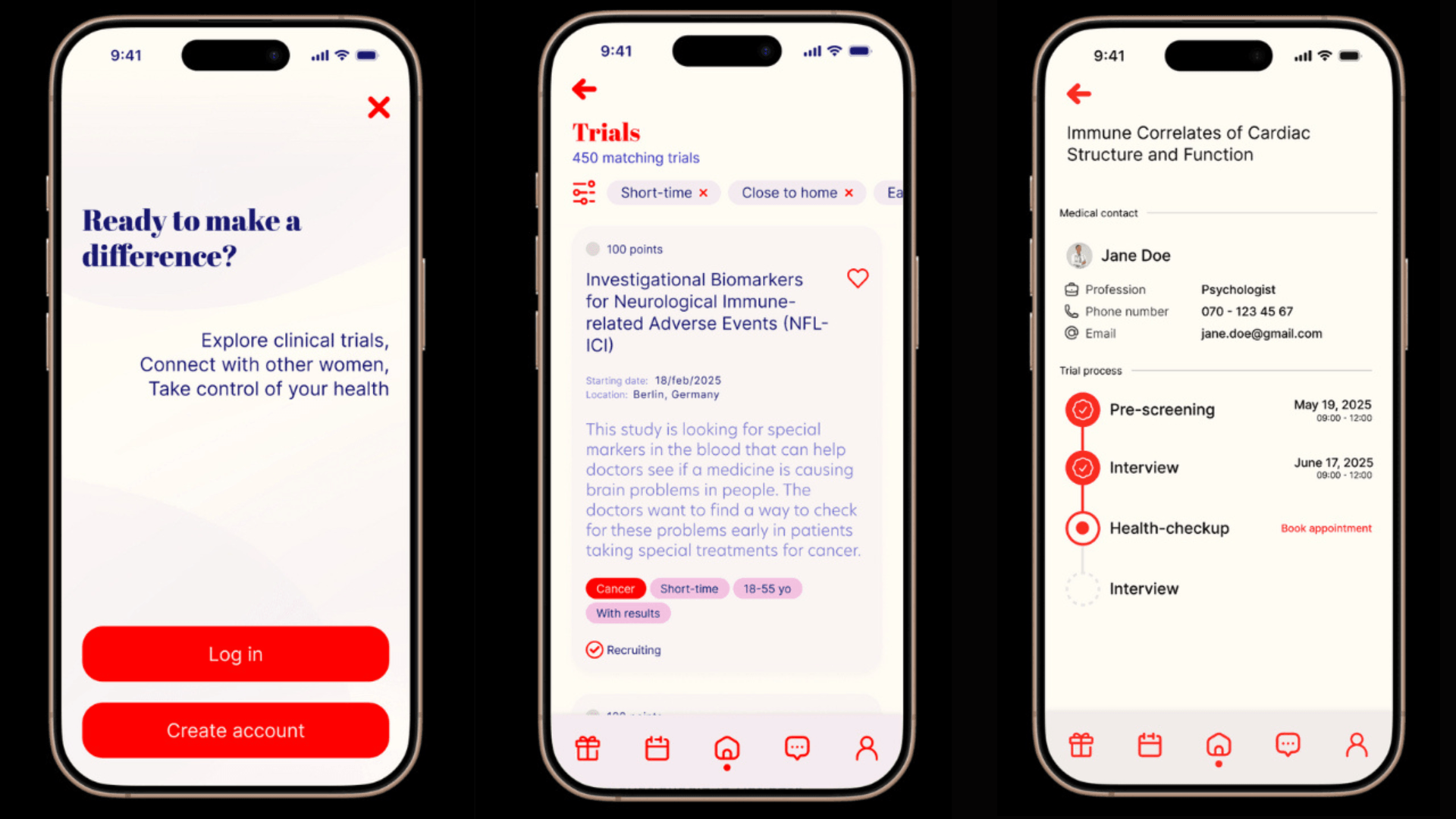
TrialMe is a platform designed to make participation in clinical trials accessible, transparent, and less intimidating — particularly for women, who have historically had very poor experiences with medical research.
The platform is the brainchild of Hanna Kalesse, who I spoke with at Slush.
“It completely knocked me out for nine months”
Kalesse is a medicinal chemist, so she’s been aware of the gender health gap for a long time.
“It’s something we learn during our studies. But what really pushed me to act was experiencing it personally.”
Kalesse was prescribed medication that she was told would take about two weeks to adjust to, after which she could continue studying and working as normal.
Instead, she shared, “It completely knocked me out for nine months. I couldn’t ride a bike, couldn’t read, and couldn’t even have proper conversations. It turned my life upside down.”
It all started with a hackathon
Around this time, there was a women’s health hackathon at Sahlgrenska Science Park. Her best friend wanted to participate but didn’t have a concrete idea. Kalesse told her: "I know a problem, and I have an idea."
“We joined together, placed in the top three, and Diana from Daya Ventures encouraged us to turn it into a real company. That was the starting point for TrialMe.”
Why underrepresentation distorts diagnosis, dosing, and care
The exclusion of women from drug trials and medical research has a significant impact on the evidence for prevention, diagnosis, and treatment of health conditions, especially those that disproportionately affect women, from autoimmune diseases and migraine to chronic pain and mental health disorders, Alzheimer’s disease, and cardiovascular disease.
Despite their prevalence and burden, these conditions frequently lack robust, sex-stratified clinical data, leaving critical gaps in how they are diagnosed, assessed, and treated.
When differences in women’s biology, hormones, and risk profiles are not factored in, critical variations in diagnosis, symptom presentation, treatment, and optimal dosing go unrecognised. The result is higher rates of misdiagnosis, suboptimal care, and adverse drug reactions among women.
The exclusion of childbearing age women a barrier to women's health research
One of the biggest problems is the exclusion of women of childbearing age from research, a sizable percentage of the population. How reproductive-age policies still sideline women in clinical trials
Historically, women of reproductive age were routinely excluded from trials due to concerns about fetal risk and assumptions that hormonal cycles made women “too complex” to study.
According to Kalesse, in Spain, for example, women of childbearing age are broadly excluded from clinical trials. In Sweden, where we’re starting, the approach is different.
“Here, participation is possible with safeguards — for example, pregnancy testing at each visit or using birth control. In my case, birth control wasn’t an option due to severe side effects, so instead I had to clearly state that I would do my best not to become pregnant and undergo regular pregnancy tests.
The blanket exclusion of women “just in case” remains a major barrier to building accurate medical evidence.”
Regulation is still catching up
Shockingly, it wasn't until 1993 that federal legislation mandated the inclusion of women and minorities in all NIH-sponsored clinical research, and that study designs allow analysis of sex-based differences. However, this does not apply to any research with other sponsors.
It wasn’t until 2022 in Europe — you read the right — that the EU Clinical Trials Regulation was amended to explicitly require trial populations to reflect the demographics of those likely to use the medication, including gender balance, “unless otherwise justified in the protocol.”
Sponsors must justify any lack of representation and can no longer omit women (or other demographic groups) without a scientific or ethical rationale.
That said, they can still claim they were unable to recruit an equal percentage of female trial participants to men.
The hidden cost of recruitment
Recruitment is one of the biggest bottlenecks in clinical research – around 80 per cent of trials fail to recruit on time. Recruitment typically accounts for around 40 per cent of a clinical trial’s total cost.
A considerable amount of that budget is wasted on inefficient screening — hundreds of phone calls to end up with a handful of eligible participants.
Initially, TrialMe reaches out directly to research organisations and trial sponsors.
Kalesse explained that if sponsors know they can access a verified, engaged community of women through TrialMe, they can significantly shorten recruitment timelines.
"Once we prove this through pilot studies, sponsors will increasingly come to us. Many women are still afraid of clinical trials, and that fear is rooted in real history.
My role is focused on education and trust-building: explaining what the gender health gap is, how it affects us, and what clinical trials actually look like in practice.”
Testing the system from the inside
To increase transparency, Kalesse is personally participating in a documented clinical trial, "so women can see what the process really involves — not just the theory.”She searched for a clinical trial for a medication she knew — as a chemist — would likely suit her better.
“It was almost impossible,” she revealed.
"“Websites were outdated, trials weren’t recruiting anymore, and many never replied at all. Eventually, I did find a trial, but it took an enormous amount of energy at a time when I was already unwell.
That frustration became core market research for TrialMe.”
The TrialMe mobile app is designed to be extremely simple. Instead of browsing endless listings or swiping like a dating app, users receive notifications when a clinical trial is suitable for them.
“The idea is to remove friction,” shared Kalesse.
“Women already carry a lot of cognitive and emotional load — participating in research shouldn’t add more.” TrialMe digitises pre-screening through the app, filtering out ineligible participants early. We don’t touch participant compensation — we make recruitment dramatically more efficient. That helps trials run faster, reduces costs, and ultimately brings safer, better-tested treatments to market sooner."
Through TrialMe, participants can earn points in exchange for things like gym memberships or therapy sessions. Even without points, participants gain early access to new women’s health products and discounts.
Why one dose doesn’t fit every date
TrialMe’s first pilots focus on menstrual cycles and medication interactions. According to Kalesse, there’s growing evidence that medication effects and side effects correlate with different phases of the menstrual cycle — but inadequate research.
“We’re starting with depression and anxiety medications, which are already known for having side effects linked to hormonal changes.
In my own case, antidepressants dramatically intensified my menstrual symptoms, including severe suicidal ideation just before menstruation. In an ideal world, this could lead to phase-specific dosing — adjusting medication depending on where someone is in their cycle. If the correlation is real, it would mean that many existing drugs need to be re-evaluated.”
From at-home diagnostics to digital health
However, Trialme is not only about drug trials. Many health products and services — including digital health tools — require testing, and women should be represented there too. For example, according to Kalesse, at-home endometriosis tests or at-home mammography tools still need validation.
“TrialMe allows women to discover and test these products, which are often hard to find once they reach the market because they come from small companies.”
TrialMe is looking global. Women aren’t the only underrepresented group in research — many minorities are systematically excluded.
"If we want medical evidence that actually reflects real populations, we can’t limit ourselves to Europe. That said, we’re starting with smaller countries to get the model right before expanding internationally,” explained Kalesse.
Clinical trials aren’t only for the ill
Further, clinical trials aren’t only for people who are ill. Healthy volunteers are just as important — especially when testing new health products and services.
“Most importantly, participation helps close the gender health gap. This isn’t a question of whether it should be solved — it’s a question of who will solve it first. And we believe TrialMe can lead that change.”
TrialMe recently won a global pitch competition organised by Tesla Ventures ahead of Web Summit. As a result, its been invited to Ireland for a one-week programme and received a year-long mentorship — led by women. Its also currently part of two incubator programmes supporting its early growth.
For decades, women were missing from the data that shapes modern medicine. TrialMe is betting that rebuilding clinical research around real populations — not theoretical defaults — is how that gap finally closes.


Would you like to write the first comment?
Login to post comments