Conceptive provider Choice today announced a partnership with French deeptech SilMach to provide a new form of contraception for women. The hormone-free approach is powered by a new microvalve that opens and closes the fallopian tubes to achieve contraception. It aims to provide greater reliability and addresses ongoing concerns about the impact of contraceptive pills & IUD’s on long-term well-being.
I spoke to Peter van de Graaf, CEO of Choice and Pierre-François Louvigné, SilMach’s CEO, to learn more.
Based in Eindhoven, Choice was founded in 2018 to develop a superior contraception alternative to current methods, which have not fundamentally changed in 60 years. This is necessary as worldwide 40 per cent of pregnancies are still unintended. Rethinking fertility:
'What if you could just switch it off?'
While thinking about how to address this, something else became clear to van de Graaf; women were increasingly fed up with hormones.
“There was growing pushback against the available options. I started to imagine a different approach — what if you could simply be infertile until you want to be fertile? Essentially, close off your reproductive system when you’re not intending to use it.”
Targeting the fallopian tubes — nature’s control point
After many conversations with gynaecologists and scientists, Choice determined that the fallopian tubes are the optimal place to intervene.
“You can’t close off the cervix because fluids need to pass, but the fallopian tubes are a natural point of control. The basic idea was to make a removable obstruction in the most suitable place in the reproductive system. So we set out to develop a wirelessly controlled valve that could be implanted once, like an IUD, and left in place permanently,” he shared.
However, the fallopian tubes are extremely small — about 1.6 mm in diameter, roughly the size of an uncooked rice grain.
That raised the key question: how do you find or build a motor small enough to fit and operate there?
According to van de Graaf, the answer came from a watchmaker friend:
“I asked him if he knew anyone who could make very small motors, and he mentioned he’d read about SilMach. That’s how I reached out to them.”
Meet SilMach: the MEMS pioneer powering aerospace, defence… and now contraception
SilMach (Silicium Machinery) is a company I’ve had on my radar for some time. It was founded in 2003 in Besançon around a strong conviction: to bring to life the first hybrid MEMS (Micro-Electro-Mechanical Systems) micromachines. SilMach designs, develops, manufactures, and integrates high-performance MEMS microsystems, including micromotors for mobile and connected systems or energy-free micromechanical sensors.
According to Louvigné:
“ Innovation is our DNA — all we do is imagine and develop the technologies of the future.
We developed a new type of micromotor for the watch industry, which is already being used in commercial watches. There’s nothing else comparable to it.”
Having won a number of innovation contests and awards, SilMach's patented PowerMEMS and ChronoMEMS solutions and technologies have endless application fields for high-stakes industrial markets such as aerospace, defence, transportation, medical, and of course, watchmaking.
SilMach has been contracted by France’s Defence Procurement Agency to integrate its ChronoMEMS sensors into ballistic plates used in soldiers’ body armour. The goal is to improve soldier safety by enabling real-time integrity checks of armour, reduce maintenance costs, and ensure traceability of protective gear in the field.
The company was awarded a “Best Of Innovation” award, the highest distinction of the CES Innovation Awards in the “Embedded Technologies” category in 2024.
“One of the most challenging implant technology projects ever attempted”

Louvigné shared that “when Peter approached us, we saw the project as one of the most challenging implant technology projects ever attempted."
"Initially, we thought the motor might be too small to move the valve. But after calculations and simulations, we realised it was technically feasible.
We redesigned the micromotor to fit inside a rice-grain-sized implant and proved it could activate the valve wirelessly.
We then moved from lab simulations to prototyping, and today we’ve demonstrated the full integration of the micromotor with Peter’s valve. It’s still ongoing, but the technical foundation is there.”
Louvigné admits that powering the device was one of the biggest engineering hurdles.
“Getting wireless energy 10 to 20 centimeters deep into the body is really hard. A pacemaker is just under the skin, but our implant sits much deeper, with an extremely small antenna. Long waves travel through the body better, but our short antenna means short waves — the worst scenario.
We worked closely with TU Eindhoven, formerly the Philips University, and one of Europe’s best wireless professors. We also collaborated with some incredibly talented female students, who were very inspired by the project. “
Together, they found a way to deliver power deep into the body despite the constraints. Following three years of development,
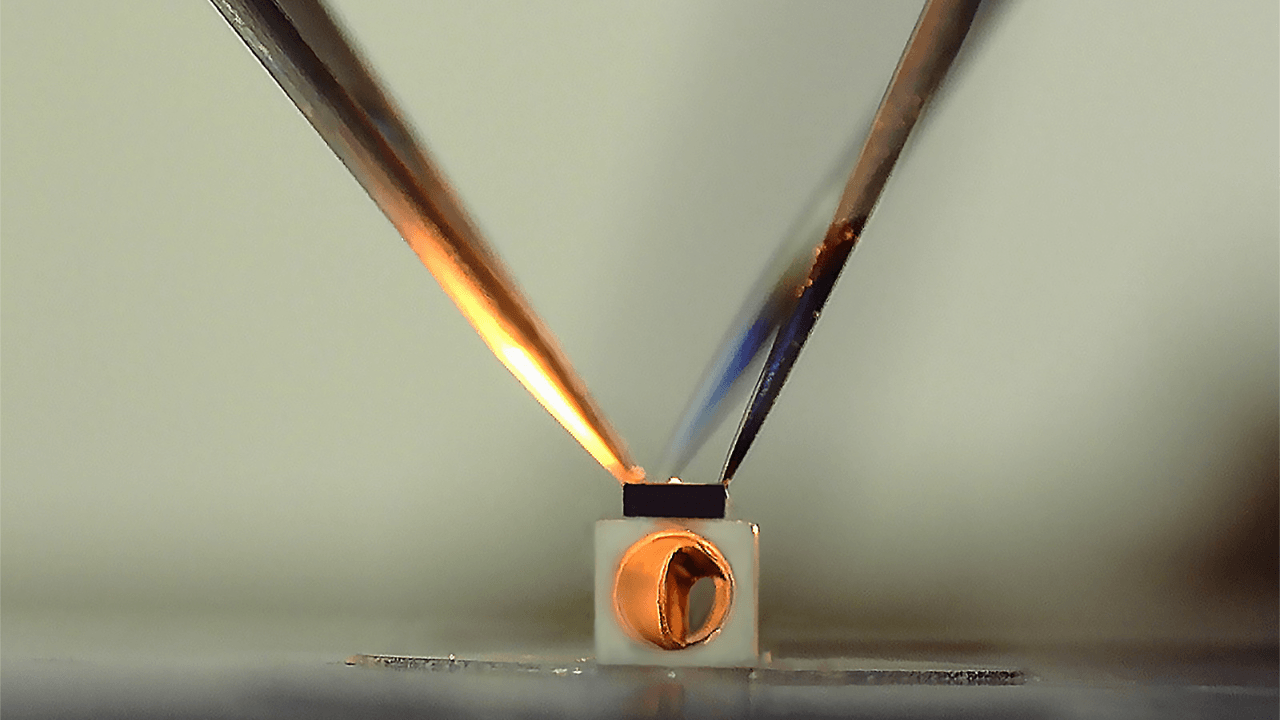
SilMach successfully developed a custom micromotor in line with Choice’s specifications, reliably opening and closing the valve. Measuring 10 x 1 x 0.1mm, the micromotor is integrated into a chip and works on electrostatic actuation, wherein positive and negative voltages are applied on microscopic, comb-like structures. The resulting attraction or repulsion moves a mechanical gear that operates the valve.
The available energy is converted into movement in very small steps with almost no heat generation (video). The small increments also mean the energy requested can be delivered in small packages, ensuring precise performance and extending the longevity of the implant.
The prototype antenna is wrapped around the implant, which is the size of a rice kernel. Power transmission is slow but reliable. And that’s fine, shared van de Graaf— this isn’t something you use daily.
“If you want, say, two children in your life, you’ll activate it four times total — to switch from infertile to fertile and back again”
Comfort, control, and reversibility
Choice provides a reliable and reversible form of contraception, which is hormone-free, pain-free and requires minimal ongoing care unlike copper IUDs, which are painful to insert and cause heavier periods, or hormonal implants, which fail to rest in the place they were initially inserted.
It reduces the risks associated with exposure to hormones and provides a valuable option for women having suffered from, or in remission from hormone-dependent cancers (such as breast cancers), who can no longer use traditional contraception.
Switching fertility on or off becomes a 30-minute clinic visit
In terms of utility, van de Graaf predicts that the external transmitter will be located in a medical clinic, not at home.
“The antenna is large and expensive, and since you only use it a handful of times in your life, you’d probably lose it between uses anyway.”
The process would be: you visit the clinic, place the external antenna on your abdomen, and wait about 30 minutes while the valves turn.
“Because the SilMach motor is extremely energy-efficient — converting almost all electrical energy into mechanical energy — we can power it slowly, one step at a time.
We tested this with women, and they were comfortable with the idea. This is a significant, meaningful moment in their lives — deciding to become fertile or infertile — so waiting half an hour is acceptable.”
Why do women have to bear the responsibility for birth control?
According to van de Graaf, there are social and biological reasons. Socially, women are the ones who bear the consequences of unwanted pregnancies.
“Even when women say they want men to take responsibility, research shows they don’t trust men with contraception outside of long-term, stable relationships. In many cases, women simply want the power in their own hands.”
Further, biologically, male contraception is vastly more complex.
An 18-year-old male produces 100 million sperm a day, compared to one egg per month — a three-billion-fold difference.
In addition, studies in men, such as the Chinese Gossypol trials, tried to develop male contraception, found that after five years of sterilisation, around 50 per cent of men become permanently infertile due to immune system changes.
“So from both angles, focusing on women makes the most sense.”
Choice is originally targeting developed markets. The implant will cost around $2,500. Over a woman’s lifetime, this system can compete with or even undercut the total cost of other contraceptive methods.
Countering conservative backlash with open-source resilience
I wondered about the impact of health initiatives such as Choice, given the conservative push back in countries like the US where radical pro-life groups — and the Republicans — are already trying to reduce (or even in the future ban all contraception, calling the pill and IUD “abortive.” despite broad opposition from the American public. The US government also confirmed plans to destroy millions of dollars' worth of taxpayer-funded contraceptives meant for women in low-income countries.
“We’re very aware of these issues,” stressed van de Graaf.
“Especially as in some autocratic societies, a technology like this could theoretically be misused to control fertility.
“Our solution is to make the external antenna design open source. Anyone with basic electronics knowledge and a soldering iron could build it. This ensures the technology remains under women’s control, not governments’.”
The device is funded primarily through angel investors. A few months ago, Choice launched a crowdfunding campaign,
Supported by an EU Eurostars Innovation Subsidy worth €450 000 over 2022-2025, the first phase of clinical testing will commence Q4 2026, for a projected launch around 2032.
van de Graaf shared:
“If we don’t get everything perfect in the first generation, that’s okay. I compare it to the Wright brothers — we just need to get this damn kite off the ground.
If we succeed, it will be revolutionary for the contraceptive world, and future generations of the product will become cheaper and more widespread.”


Would you like to write the first comment?
Login to post comments