IV treatment is one of the most traditional responsibilities of the nurse personnel provided to 90 per cent of hospitalised patients. Yet traditional IV poles significantly limit patient mobility, tethering them to a fixed location and making everyday activities like walking, using the restroom, or participating in physical therapy challenging, therefore increasing the nursing workload.
IV poles also pose safety risks — unstable structures can lead to falls, and manoeuvring them through crowded hallways, elevators, and hospital rooms increases the likelihood of patient and staff accidents.
Additionally, their bulky design contributes to clutter in high-traffic areas, creating physical obstacles. IV poles rely on gravity to administer fluids, and movement or height imbalances can disrupt the flow, necessitating frequent nurse intervention.
But now, a Danish startup has created the future of IV devices.
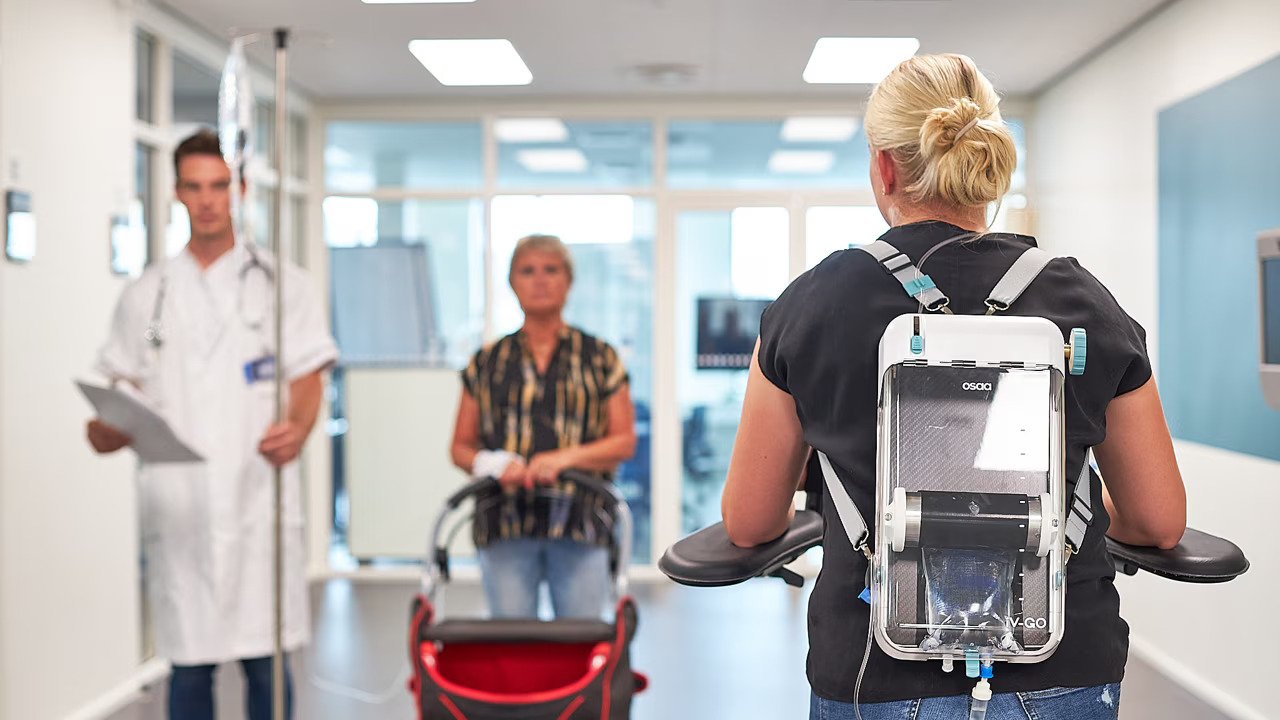
OSAA Innovation has developed iV-GO, the world's first fully portable and 100 per cent mechanical IV pump. It liberates individuals from the constraints of hospital beds and IV poles, allowing for treatment "on the go"— whether in hospitals, at home, or even outdoors.
I spoke to Ahmed Hessam, CEO and founder of OSAA Innovation, and QA/RA manager Alexei Cernenco to learn more.
Hessam is the inventor of iV-GO, which was inspired by seeing a child playing outside while her mother held her IV bag:
The initial concept began as a simple idea: a backpack with an IV pole sticking out of it. That let a young girl stay active and play while still receiving treatment. Over the following years, the idea evolved.
"One day, while casually fiddling with a pen, it broke, revealing a spring inside.
Springs are fascinating because they generate energy when compressed and then release it as they push back.
This mechanical principle became the foundation for our first prototype, which we called iV-GO."
The key advantage of iV-GO is its fully mechanical operation. Unlike electronic devices, it requires no batteries or charging, making it reliable in any environment. This feature ensures uninterrupted functionality, regardless of the setting.

The pump weighs less than two kilograms, even with a full IV bag, and allows patients to move freely during treatment. With a lightweight design and adjustable straps, it can be attached to a wheelchair or worn as a backpack while maintaining consistent pressure and infusion.
The device has garnered international attention as the company has won a variety of awards, including the Danish Venture Cup (2017), the Danish Design Award (2021), Red Dot Design (2022), Good Design (2023), Australian Good Design (2024) and German Design Awards (2025).
Due to complex procurement processes and lengthy sales cycles, medtech startups often face challenges selling their products to healthcare providers. According to Hessam, OSAA Innovation has successfully gained traction within hospitals by involving them early in development.
"With iV-GO and all our devices, we conduct extensive field research. We actively engage with nurses, asking what they need and want," Hessam explains.
"One of the most significant issues we've observed with highly technological solutions is their complexity, which leads to usability errors.
To address this, we regularly return to hospitals to gather feedback on new features, ensuring our designs are intuitive and practical. When nurses feel involved in the process, they're more likely to adopt and continue using the device once it's launched."
Since the introduction of the iV-GO, the company has expanded to a suite of products, including connected iV2GO and iV2Vita with IoT monitoring, smart sensors and dashboard software, and the iV4GO Pro and iV4Vita Pro — an advanced infusion rate monitor and regulator designed to enhance the safety and efficiency of intravenous therapy. It includes treatment monitoring, a fall detection sensor, cloud connectivity, and an intuitive dashboard that offers reports and statistics.
An optional cloud-based system iV-View enables hospital staff to monitor multiple devices across wards, and access vital information on active treatments.
It notifies staff when treatment is nearing completion, enhancing workflow efficiency and patient care, while the digital record-keeping feature ensures accurate documentation, simplifying patient care and record management while ensuring full compliance with treatment plans.
The company is also developing an IV Warmer for transfusions that can help prevent hypothermia and improve patient comfort during IV therapy.
Cernenco explained that the iV-GO suite of iV devices is distinct because it is designed on an open system, which gives health care providers a lot of flexibility.
"Because all the systems are closed, staff need to use different tubing for different machines, and the ability to line interchangeably has attracted huge interest from nurses."
Further, the software is designed as a standard architecture that can be easily integrated into many different systems globally from Europe to Australia.
The company has attracted significant interest from emergency service personnel and disaster relief service providers.
OSAA complies with ISO 1345 medical device standards, and the iV-GO is certified in Europe, Australia, Saudi Arabia, and the United States, with additional devices currently undergoing regulatory approval processes.
In terms of the security of connected devices, while IV pumps security vulnerabilities in the 2010s were disclosed by white hat hacker researchers, there have been no reported incidents of malicious hacking of pumps.
Cernenco emphasised the security-first approach, explaining that the community has both devices with one-way communication, "which makes them very secure and difficult to interfere with," and heightened cybersecurity measures for devices with two-way communication to ensure safety.
OSAA Innovation also prioritises transparent communication about safety features, such as guardrails, to build trust and confidence among clinicians.
"It's essential for healthcare providers to feel secure when using our devices," Hessam adds. This commitment to usability and security has played a significant role in OSAA's growing success in medtech.
We're both developing and simultaneously certifying the new devices, which are next-generation devices. That will be, of course, our focus from now on. We always have a future."
OSAA Innovation expects to start manufacturing new devices in April and launch them in Q3 2025.
The company has raised €5.5 million in investment.
Lead image: OSAA Innovation.


Would you like to write the first comment?
Login to post comments