Today, a coalition of 33 digital health organisations from across Europe, including industry associations and startups from France, Germany and other EU member states, has published a joint statement urging European decision-makers to establish a convergent and pragmatic evaluation framework for digital medical devices (DMDs) starting in 2026.
The signatories warn that Europe’s position as a global leader in digital health is at risk, not due to a lack of innovation, but ongoing regulatory fragmentation.
The statement asserts that as long as each country follows its own approach, the European digital single market remains theoretical. This patchwork system leads to duplication of effort, prevents companies from scaling across borders, and delays patient access to treatments that have already been proven safe and effective. More critically, it undermines Europe’s ambition to achieve technological sovereignty and compete on equal terms with global powers.
“The race for European Technological Sovereignty is happening now. We need access to a unified European digital health market - now.”
Remote monitoring and digital therapeutics thrive locally, but stall at EU level
With its 450 million citizens, the European Union (EU) faces numerous healthcare challenges, including an ageing population, an increase in the prevalence of chronic illnesses, and a growing shortage of healthcare professionals.
A new generation of European digital health startups has already demonstrated its potential to address these challenges through robust clinical studies and technical certifications in their home markets.
The adoption of their solutions is well-established among patients and healthcare professionals (HCPs). Hundreds of thousands of patients have already used remote patient monitoring (RPM) or remote therapeutic monitoring (RTM) in France, or digital therapeutics (DTx) in Germany, following a medical prescription.
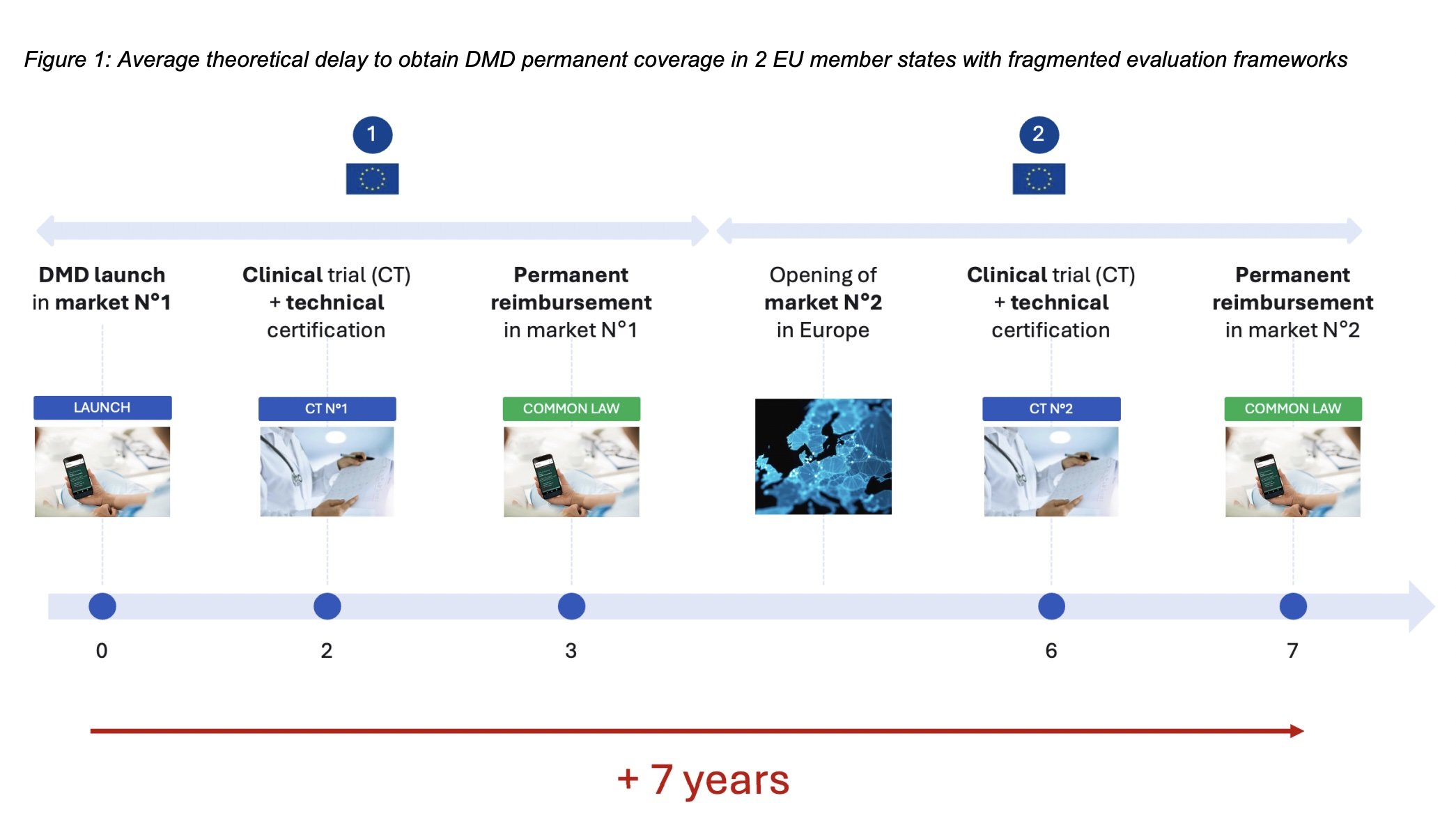
However, according to the statement, diverging evaluation frameworks across EU member states prevent these solutions from scaling from one country to another and from accessing sustainable economic models.
The statement contends:
“In the context of intense global competition and rapid technological advancement, this fragmentation threatens the survival of the European digital health industry and undermines the EU’s technological sovereignty.
It also leads to unequal access for patients, healthcare professionals, and payers across Europe.“
Fragmented rules could leave digital therapeutics and AI health tools obsolete
The coalition advocates for a unified EU evaluation framework with consistent clinical and technical criteria.
It would not only enable the emergence of strong European players capable of competing globally but also safeguard Europe’s technological sovereignty, but support the integration of digital health solutions into the upcoming European Health Data Space (EHDS).
Without the widespread adoption of these solutions across the EU, the EHDS risks remaining as a theoretical construct with limited operational impact.
Next steps
Starting in 2026, particularly in France and Germany, the largest healthcare markets in Europe, the coalition urges for alignment on:
- Technical requirements for DMDs certification
- Clinical evaluation criteria for Digital Therapeutics (DTx), Remote Patient Monitoring (RPM), Remote Therapeutic Monitoring (RTM) and AI-powered health solutions
- Operational procedures and methodologies for DMDs reimbursement pathways.
The statement asserts:
“We also call for the future European evaluation framework to be truly pragmatic, ensuring that healthcare innovations can access the EU-wide market within a reasonable timeframe (2 to 3 years maximum) after their launch in their home country. Beyond this timeframe, such technologies risk becoming obsolete.“
Signatories include:
Industry associations:
- Frédéric Girard, France Biotech
- Marianne Tordeux Bitker, France Digitale
- Guirec Le Lous, MedTech in France
- Anne Sophie Geier, Spitzenverband Digitale Gesundheitsversorgung (SVDGV)
Companies:
- Matthieu Lamy, Ad Scientiam
- Guillaume Ploussard, AIMED2
- Boris Lévêque, Axomove
- Felix Köhler, Cara Care
- François-Guirec Champoiseau, Cureety
- Stanislas Niox-Chateau, Doctolib
- Nadine Rohloff, Endo Health GmbH
- Fabien Watrelot, ENSWEET
- Emeline Hahn, Fizimed
- Stan Sugarman, GAIA AG
- Hannes Klöpper, HelloBetter
- Arnaud Rosier, Implicity
- Manuel Thurner, Kaia Health
- Alexia Adda, Klava
- Jens Nörtershäuser, Kranus Health
- Pierre-Camille Altman, MDHC
- Philip Heimann, Vivira Health Lab


Would you like to write the first comment?
Login to post comments